XSENSOR pressure measurement validated by University of Salford
XSENSOR
University of Salford’s School of Health and Society conducted a review of the XSENSOR Intelligent Insoles‘ validity and reliability across different pressures, durations of load and contact areas, determining their aptness to address different research questions or clinical assessments.
Background
In-shoe pressure measurement systems are used in research and clinical practice to quantify areas and levels of pressure underfoot whilst shod. Their validity and reliability across different pressures, durations of load and contact areas determine their appropriateness to address different research questions or clinical assessments. XSENSOR is a relatively new pressure measurement device and warrants assessment.
Research question
Does the XSENSOR in-shoe pressure measurement device have sufficient validity and reliability for clinical assessments in diabetes?
Methods
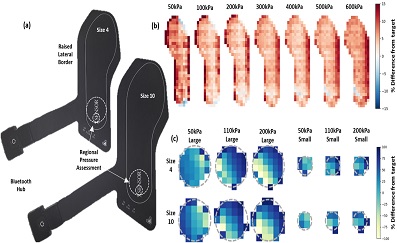
Two XSENSOR insoles were examined across two days with two lab-based protocols to assess regional and whole insole loading. The whole insole protocol applied 50–600 kPa of pressure across the insole surface for 30 seconds and measured at 0, 2, 10 and 30 seconds. The regional protocol used two (3.14 and 15.9 cm2 surface area) cylinders to apply pressures of 50, 110 and 200 kPa to each insole. Three trials of all conditions were averaged. The validity (% difference and Root Mean Square Error: RMSE) and repeatability (Bland Altman, Intra-Class Correlation Coefficient: ICC) of the target pressures (whole insole) and contact area (regional) were outcome variables.
Results
Regional results demonstrated mean contact area errors of less than 1 cm2 for both insoles and high repeatability (≥0.939). Whole insole measurement error was higher at higher pressures but resulted in average peak and mean pressures error < 10%. Reliability error was 3–10% for peak pressure, within the 15% defined as an analytical goal.
Significance
Errors associated with the quantification of pressure are low enough that they are unlikely to influence the assessments of interventions or screening of the at-risk-foot considering clinically relevant thresholds. Contact area is accurate due to a high spatial resolution and the repeatability of the XSENSOR system likely makes it appropriate for clinical applications that require multiple assessments.
About XSENSOR’s X4 Intelligent Insole System
Capture dynamic high resolution, in-the-field lab quality data with XSENSOR’s fully wireless X4 Intelligent Insole System.
The X4 Intelligent Insole System provides the most accurate plantar pressure and gait data available in any test environment.
The X4 system offers ease of use, and assurance of quality test data, for biomechanics professionals. It offers high-speed recording in compact and discreet on-shoe wireless electronics, enabled by Bluetooth, which are paired with durable high-resolution sensors.
Ultra thin at <2mm. Sensors conform to the footbed of the shoe and are virtually undetectable to the wearer.
Advanced functionality. Complete analysis for research and performance using XSENSOR’s X4 Pro Foot & Gait software.
Accurate, reliable and repeatable. 3% or less full-scale error after 100,000 loading cycles.
Contact us
Summit Medical and Scientific are the UK sales partner for XSENSOR.
For more information, please contact us, send us an email or give us a call on 01372 459863.
You can also follow us on Facebook, Twitter, LinkedIn, and sign up for our newsletter.